Have you ever pondered the question, “Where is most of the mass of an atom located?” It is a fascinating inquiry that takes us on a journey deep into the heart of matter itself. Atoms are the fundamental building blocks of everything in the universe, from the air we breathe to the stars in the sky. Despite their importance, they are incredibly small and their secrets are hidden well beyond the reach of the naked eye.
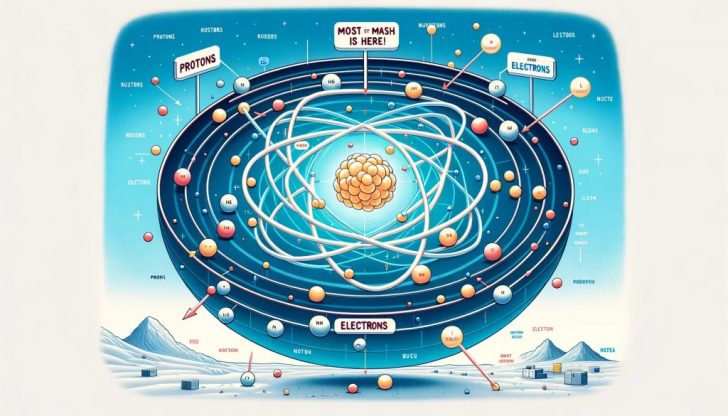
Essentially, an atom is composed of three main particles:
- Protons
- Neutrons
- Electrons.
Protons and neutrons form the nucleus at the center of the atom, while electrons orbit this nucleus at incredible speeds. Though this might sound straightforward, the distribution of mass within an atom is anything but.
The Atomic Nucleus
So, where is most of the mass of an atom located? The answer lies in the atom’s nucleus. This tiny central core is where protons and neutrons reside, and it accounts for nearly all of an atom’s mass. Protons and neutrons are collectively known as nucleons, and each has a mass approximately 1,836 times that of an electron.
Given that electrons are so much lighter, they contribute almost negligibly to the overall mass of an atom.
What is Nucleus?
This force acts like superglue, holding the protons and neutrons together in the nucleus. Without it, the positively charged protons would repel each other, causing the nucleus – and thus the atom – to fall apart. The strong nuclear force is incredibly powerful. But it operates over very short distances. This is why the nucleus is so small compared to the overall size of the atom.
If the nucleus were the size of a pea, the atom itself would be about the size of a football stadium, with the electrons zipping around the stands. This analogy helps to illustrate just how much empty space exists within an atom. It also underscores that “where is most of the mass of an atom located” is a question with a very concentrated answer: the nucleus.
Implications of Atomic Mass Distribution
Understanding where most of the mass of an atom is located has profound implications for science and technology. It is the basis for nuclear physics, which explores how changes in the nucleus can release or absorb vast amounts of energy. This principle underlies both nuclear power generation and the development of nuclear weapons.
Plus, the distribution of mass within atoms is critical for chemistry, as it affects how atoms bond with each other to form molecules and the properties those molecules exhibit.

Can the mass of the nucleus change?
Yes, the mass of the nucleus can change through nuclear reactions, such as fusion or fission. These processes can convert a small amount of mass into energy, as described by Einstein’s famous equation, E=mc^2.
How does the atomic nucleus stay together?
The atomic nucleus stays together thanks to the strong nuclear force, one of the four fundamental forces of nature. This force is incredibly strong but operates over very short distances.
What does the emptiness of atoms mean for the physical world?
The emptiness of atoms means that, on a fundamental level, solid matter is mostly empty space. However, electromagnetic forces between atoms prevent us from passing through this “empty” space, giving matter its solid feel.